



Rynd Biotech is dedicated to facilitating early detection and ongoing monitoring of urinary infections.
Our technology not only identifies symptomatic cases but also uncovers asymptomatic cases that often go unnoticed. We are working on expanding our biomarker range to include screenings for Urinary Tract Infections (UTIs), Pregnancy indicators, Urology and Oncology markers. This will allow for comprehensive monitoring of infection progression and more effective disease management.
1 Million
Asymptomatic STIs acquired every day1
Decreasing trend in US High School Students Reported condom use2
62%
2007
54%
2017
367 Million
New Urine Infections of Chlamydia, Gonorrhea and Trichomoniasis in 20201
1. “Sexually Transmitted Infections (STIs).”
2. “Youth Risk Behavior Survey Data Summary & Trends Report 2007-2017.”
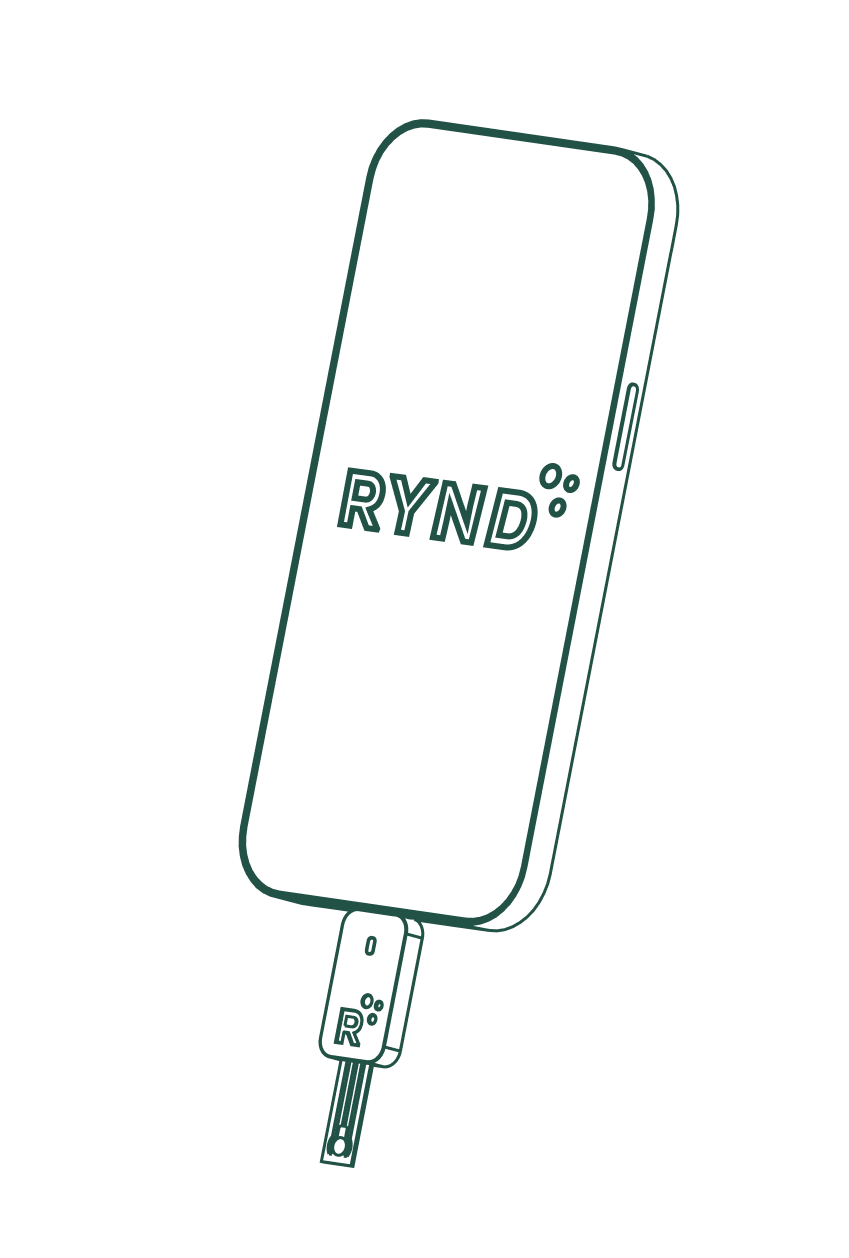
Instant, Easy, and Accurate STI Testing
Rynd Biotech introduces an innovative urine diagnostics platform that is instant, portable, accurate, and easy to use.
Our device utilizes a microfluidic chip and aptamer technology to deliver results within minutes, targeting STI pathogens such as Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.



Rynd Biotech's platform stands out against traditional PCR and NAAT lab-based tests, offering portability, rapid results, and quantitative data, unlike the qualitative results from conventional methods.
The app delivers not just a diagnosis but also automated prescriptions and epidemiological insights, revolutionizing both personal health management and public health monitoring.
The diagnostic device performs the quantification measurements and transmits the results through a USB-C connection to the app.
The test strips consist of microfluidics channels that allow the multiplex quantification of biomarkers in a single drop of urine.
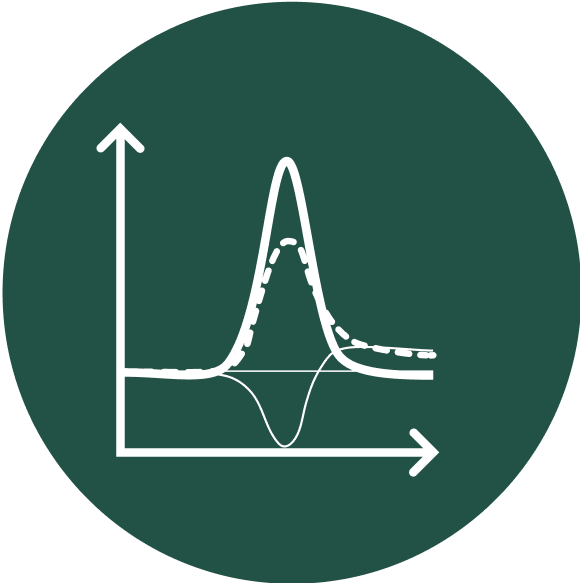
Quantification with 900X signal amplification per binding event for precise pathogen identification
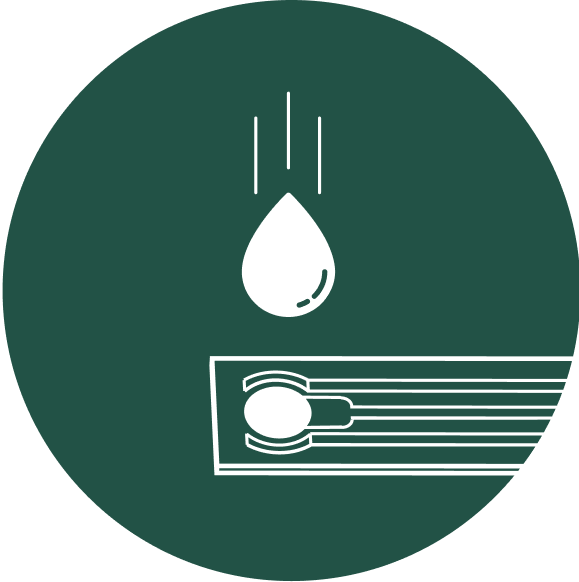
Rapid quantitative diagnosis using a single drop of urine

Telehealth services and automated prescriptions upon request
Welcome to Rynd, where we're redefining the gold standard for urine tests. Our goal is to revolutionize access to real-time health monitoring. Join us as we change the landscape of healthcare. Together, we aim to build a healthier world, foster groundbreaking innovation, and ensure enjoyment at every step.
Co-Founders
Advisors
2024
Patent Pending
STI Testing - First Prototype
FDA Submission
2024
-
2025
FDA Approval
Research In UTIs, Pregnancy, Urology And Oncology
2026
Application To Research Fields
Leading Platform In Urine Diagnostics
© 2023 Rynd Biotech. All rights reserved