



Rynd Biotech is dedicated to transforming bladder cancer care with a rapid urine-based diagnostic test using a patented 4-biomarker panel. Our diagnostic platform enables accurate, non-invasive detection and monitoring, reducing the number of painful cystoscopies.
Our Goals:
• Detect the most aggressive high-grade tumors early and reliably
• To improve patient outcomes and reduce cystoscopies healthcare burden
• Enable point of care and at-home diagnostics
• Increase access in high- and low-resource settings
From detection to lifelong monitoring, our mission is simple:
Give every bladder cancer patient and clinician the confidence of fast, painless, accurate answers.
70%
of bladder cancer patients experience recurrence1
bladder cancer survivors in the U.S. alone3
>740,000
Most expensive
cancer to manage per patient3
1. Mossanen M, Gore JL. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol. 2014 Sep;24(5):487-91.
2. https://seer.cancer.gov/statfacts/html/urinb.html
3. https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html

Rapid, Painless, and Accurate Urinary Diagnosis
Our platform leverages the sensitivity of electrochemical quantification with the specificity of proprietary monoclonal antibodies to deliver results in minutes. It pairs with a portable reader and companion app to guide recurrence monitoring and early detection.
Key Results:
Through mass spectrometry discovery and Western blot initial validation in 200 patients, we have identified 4 novel, patented urine biomarkers. These biomarkers demonstrated 100% sensitivity for high-grade tumors.
Today:
We are developing a multiplex lateral flow immunoassay (LFA) that delivers rapid, non-invasive results for bladder cancer recurrence monitoring at the point of care ensuring an easy entry into the market.
Tomorrow:
Our next-gen electrochemical platform designed to scale across the full spectrum of urologic oncology care:
• Higher sensitivity and real-time quantification
• Hematuria triage and population screening
• Expansion to prostate, kidney, and other urologic cancers
• Home use with digital connectivity for global scalability



Unlike cystoscopy and cytology, our platform offers a non-invasive and rapid solution with quantitative biomarker data.
The app provides real-time risk scores, tracks patient history, and supports clinical decision-making.
The USB-C reader delivers immediate results directly to the app, empowering both clinicians and patients.
Our test strips allow the quantification of four biomarkers in urine with high sensitivity and specificity.
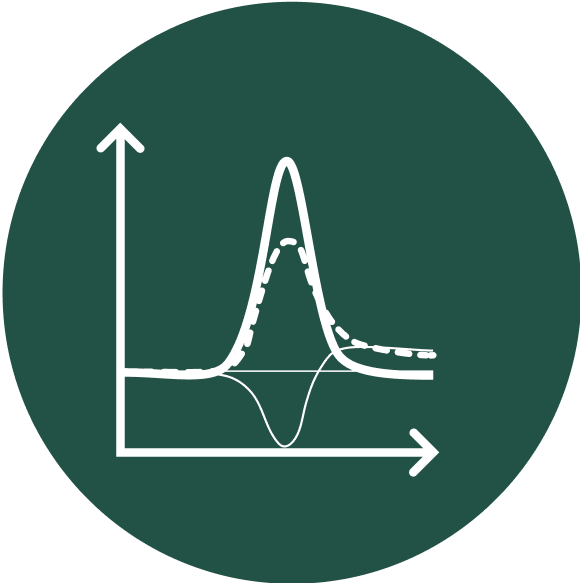
Square-wave voltammetric for accurate quantification
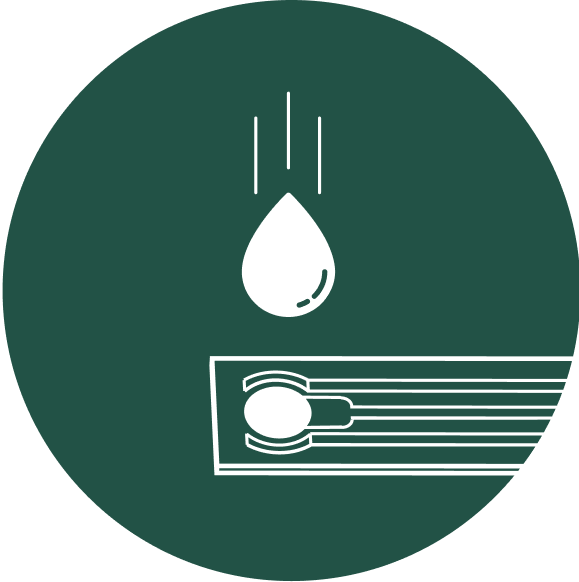
Rapid diagnosis using a single drop of urine

Telehealth services and online monitoring
Welcome to Rynd Biotech, where we're redefining bladder cancer detection. Our multidisciplinary team of molecular scientists, physicians, and regulatory experts is committed to advancing non-invasive diagnostics. From lateral flow strips to a future electrochemical reader, we're building a platform for current and future urinary disease detection.
Team
Advisors
© 2025 Rynd Biotech. All rights reserved